BIOAVAILABILITY
&
BIOEQUIVALENCE
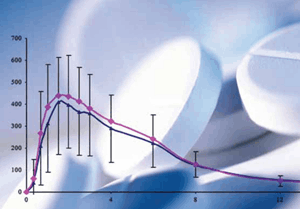
Bioavailability (BY) is the rate and degree of absorption of the active substance through the pharmaceutical form and reaching its site of action in the body. The main parameters used to measure the bioavailability of an orally administered drug are “area under the plasma concentration-time curve (Area Under the Curve, AUC)” and “plasma peak maximum) drug concentration (Cmax).
Bioequivalence (BE) is that two different drug products that are pharmaceutical equivalent (for example, both tablets / capsules) have similar bioavailability and hence therapeutic effects after administration at the same molar doses.